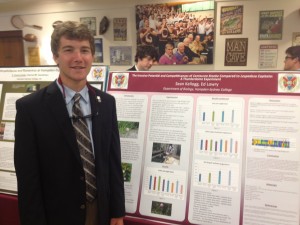
Hampden-Sydney’s summer research students presented their work in a public poster session as part of the College’s “C-Day” activities. The session gave the students the opportunity to present their research to professors, fellow students, and other members of the Hampden-Sydney community. The Biology Department was pleased to be represented by 11 students presenting ranging from ecology and organismal biology to genetics and molecular biology.
Author Archives: admin
A summer at Duke Marine Lab with Tom Drury ’14
Tom Drury ’14 joined the ranks of Hampden-Sydney students who have spent a summer or academic year semester at the Duke Marine Lab. Tom took a course called “Conflicts in Global Fisheries” and was able to research the conflicts arising between commercial fishermen and sea turtle conservation. He also studied the effects of recreational fishermen on the North Carolina coast, especially in Carteret County, on local fisheries. Hampden-Sydney participants at the Duke Marine Lab have augmented their classroom experience with the chance to perform aquatic biology research in a marine environment.
Welcome Dr. Stacey Gorski to the Department of Biology
I am very excited to join the students, faculty and staff at Hampden-Sydney College! I am originally from the West Coast, having been born in California and raised in eastern Washington State. I moved across the country to attend college, and after earning a B.S. in biology from University of the Sciences in Philadelphia (formerly Philadelphia College of Pharmacy), I moved to Virginia to obtain my Ph.D. in Microbiology, Immunology, and Cancer Biology from University of Virginia (UVA). It was during my time at UVA that I became very interested in lung physiology, particularly how the lung repairs itself following infection with respiratory viruses, such as influenza. Because influenza is so prevalent, we know a lot about the immune response to the virus; however, we know relatively little about the host response mechanisms that allow us to actually recover from the infection. Thus my research is primarily focused on how specific innate immune cell types contribute to pulmonary regeneration after influenza infection. I also recently returned from South Australia, where I was working for Vaxine Pty. Ltd., a vaccine manufacturer specializing in adjuvant (the immune stimulating agent in vaccines) design and efficacy. While at Vaxine I was able to apply my doctoral research to clinical trials being conducted in patients receiving a new influenza vaccine. I loved living and working ‘Down Under’ but I am so glad to be home in Virginia! I am very much looking forward to joining the biology department and becoming a part of the Hampden-Sydney community.
Yonathan Ararso ’13 Presents Cancer Research at Leadership Conference
Yonathan Ararso recently presented his award-winning research on immune suppression by melanoma at the 2013 Leadership Alliance National Symposium in Stamford, CT. His poster, entitled “Melanoma-derived Factors TGFb1 and VEGF-A Suppress Anti-tumor Immunity by Interfering with Dendritic Cell Maturation and Activation” stemmed from work conducted during his Senior Honors Project in Biology under the guidance of Dr. Kristian Hargadon, Elliott Assistant Professor of Biology. For this work, Yonathan was the recipient of the prestigious Samuel S. Jones Phi Beta Kappa Award, a $4,000 prize for the best research paper at H-SC in a given academic year. His poster at the Leadership Symposium highlighted his findings regarding the role of two melanoma-derived factors that suppress dendritic cells, a cell type whose function is critical for the induction of anti-tumor immune responses.
In August, Yonathan will begin working in the laboratory of Dr. David Lyden at Weill Cornell Medical College. Dr. Lyden is Stavros S. Niachos Chair and Associate Professor of Pediatrics and Cell and Developmental Biology at Weill Cornell Medical Center and Pediatric Neuro-Oncologist at Memorial Sloan-Kettering Cancer Center. His lab is currently studying how primary tumors secrete certain growth factors to prime specialized tissues and sites throughout the body, in effect creating a “metastatic niche” conducive to tumor cell adhesion and invasion. Yonathan also plans to apply to M.D./Ph.D. programs following this next year of research.
Premed summer preparation program with Daniel Osarfo-Akoto ’15
This summer, I participated in a program in Newark, NJ called the Summer Medical and Dental Educational Program. It lasted for about 6 weeks although only 5 of the 6 weeks was geared towards academics (The last week was saved for exams and a recruitment fair at Columbia University). SMDEP is offered at several locations, but the New Jersey site is one of the only sites that give students the opportunity to use cadavers in labs whiles having multiple shadowing experiences both in the dental and medical clinics. There were about 80 students in the program, and each student was put into a track. I was placed in a track which focused more on Anatomy and Physiology. The Physiology class was the most interesting class for me, as I could actually reason alongside Dr. Luckacs as he combined physics and biology into something quite phenomenal. The greatest challenge for me was Anatomy, as I had to be able to identify the muscles of the human body using a fresh, new cadaver. In the end, however, I was more familiar with all the different muscles in the body and how they work. There was another class called Communications which educated us on how to present to fellow students and talk about issues involving culture and health disparities comfortably.
Another beneficial part of this program was the caliber of the TAs who were assigned to each Track. They were second year med-students who could relate to us in terms of overcoming the MCAT and how to make it into medical school. There were also speakers (all of whom were doctors) who came to speak with us on how they were able to get it to Medical School, what to do to get into Medical School, and how to survive Medical School. During the 5th week, we had mock med school interviews and an informational session from the Dean of Admissions. This helped us get a general understanding of what we need to do to prepare for medical school interviews.
We were also paired with a doctor or two, to help us get a better understanding of how each field of medicine felt like. I was paired with a Transplant Surgeon, and although I didn’t get a chance to see any type of surgery at all, the Post-Ops were more than enough. I read some CT-SCANS of the lungs and saw some patients. Dr. Wilson, the transplant surgeon I was assigned to, had been in medicine for about 25 years and he was still able to pull off 32 hour shifts and not “lose his mind.”
The last but not the least of the things I was exposed to was the clinical portion of the Dental and Medical Student Final Examinations. The students had actual patients they had to deal with, and at the end of the exam, the patients would rate and give comments about the “doctor,” and that, as well as an actual assessment from the instructor organizing the test, would be part of the deciding factor if a students graduates from medical school or not.
The most amazing part of this program, however, was the friends that I made. As simple and plain as it may seem, the people who made this experience a remarkable one were the people in it. I met people with Indian, Chinese, Puerto Rican, Ecuadorian, Egyptian, Middle Eastern, and Italian ancestries, who were willing to share their life experiences and even teach me a few studying tips which I plan on implementing. There were students from William and Mary, Cornell, UPenn, Yale, and of course Hampden-Sydney (there was no student from Randolph-Macon College).
Immunology and Transplant Research at UVA with Meade Edmunds ’14
I am currently conducting research at the University of Virginia in the division of Transplant Surgery under Drs. Daniel Maluf and Valeria Mas. In regards to liver transplantation, we are studying the long-term effects of the immunosuppresive drug Tacrolimus on kidney functionality in liver transplant patients. Tacrolimus is a potent immunosuppresive drug given to allogeneic organ transplant patients that acts as a calcineurin inhibitor (CNI), which in turn inhibits T-cell activation and IL-2 production. A long-term side effect of CNIs is renal calcineurin inhibitor toxicity (CNIT), which can lead to acute and chronic renal failure. The purpose of our study is to identify specific genetic biomarkers that are upregulated in the early stages of CNIT using qPCR. We hope that, once identified, these biomarkers might serve as a universal, cost-effective standard for identifying CNIT within all transplant patients and allow transplant surgeons adequate time to alter a patient’s immunosuppressive regimen before renal failure occurs.
Alzheimer’s research at Duke with Jackson Parker ’14
This summer I have been assisting in Alzheimer’s research at Duke University Medical Center. We are studying the role that a specific mitochondrial transmembrane protein, called TOMM40, plays in the appearance of Late Onset Alzheimer’s Disease. TOMM40 is a channel protein, part of the Tom (Translocase of Outer Membrane) Complex, which plays an essential role in the importation of preproteins into the mitochondria. A genetic correlation has yet to be found between TOMM40 and APOE, considered the most influential genetic factor concerning Late Onset Alzheimer’s disease; however, mitochondrial disfunction is a recognizable effect of Alzheimer’s, which may or may not be attributed to mutated TOMM40 protein. In lab, I have performed pulverizations, homogenizations, protein determinations, and Western Blotting on both transvected mouse brains (offspring of heterozygous mice to progeny TOMM40 gene as well as a transvected human TOMM40 gene) and flash-frozen human brain tissue samples. Not only has this been a hugely educational experience, it has also been really entertaining using all of the instrumentation as well as handling liquid nitrogen despite a few instances of cold burn. My position at Duke is as a volunteer unpaid intern, but the experience is invaluable. I highly recommend to all biology majors that if the opportunity to participate in a biologic or chemical lab is available, be it paid or unpaid, take the chance because it is a prime time opportunity to participate in a collaborative environment that we are all working towards each year at H-SC.
Dr. Kristian M. Hargadon ’01 Publishes Article on Tumor-altered Dendritic Cells
Elliott Assistant Professor of Biology Dr. Kristian M. Hargadon ’01 recently published an article on tumor-altered dendritic cells in Frontiers in Immunology, the official journal of the International Union for Immunological Societies. Frontiers Journals have recently partnered with Nature Publishing Group (NPG), publisher of the Nature journal series, which has over 150 years experience as the leading publisher of groundbreaking science. Together, Frontiers and NPG are working to empower researchers to change the way science is communicated, through open access publication and open science tools. Dr. Hargadon’s article in Frontiers in Immunology highlights recent advances in the field of tumor immunology as they relate to our understanding of how tumors alter the function of dendritic cells, an immune cell type critical for the induction of anti-tumor immune responses. The article discusses not only how tumors alter dendritic cell function but also how these tumor-altered dendritic cells impact the quality of overall anti-tumor immunity. It also emphasizes recent work that has aimed to enhance dendritic cell-mediated anti-tumor immune responses through cancer immunotherapy strategies. Lastly, the article highlights questions in the field that remain to be answered as well as strategies for addressing these problems that may aid in the development of novel and improved anti-cancer treatment regimens. Dr. Hargadon’s article is the first published in what will be a series of articles from investigators all over the world in a Special Issue of this journal focusing on Tumor/Dendritic Cell Interactions.
HIV Research at Tulane with Carter Guice ’14
This summer I am working through the Undergraduate Fellowship Program at the Tulane National Primate Research Center in the division of Regenerative Medicine under Dr. Stephen Braun. As a model of HIV latency, we are studying the effects of the HIV transcription factors Tat (transactivator of transcription), LAP (liver-enriched activator protein), and LIP (live-enriched inhibitory protein) on HIV LTR promoter activity in U937-VRX494 cells in vitro. U937-VRX494 cells are cells of myeloid lineage that secrete a large number of chemokines and cytokines. They contain an HIV based vector (VRX494) with the Green Fluorescent Protein (GFP) marker gene by the HIV LTR. We are transfecting them with various concentrations and combinations of the different HIV transcription factors (Tat, LIP, and LAP) and observing the GFP expression, quantitatively measured by flow cytometry. We expect to find potential interactions of host transcription factors on HIV LTR activity during HIV latency.
Reptile viral research with Davis Carter ’15
The purpose of my research project is to sample the local population of squamates for ranavirus. The virus has caused die offs in amphibians as well as squamates. At the beginning of my research, I was specifically focused on snakes because of the lack of research that has been done on snakes and ranavirus. But, since I was seeing so many other reptiles, Professor Goodman decided it would be alright for me to collect lizards and turtles as well.
Every morning and evening rain or shine, I go out to one of the sites found around campus and check all the covers at that site. We use covers because they have been shown to be more successful in catching snakes than other techniques. There are 4 sites each with 54 covers made of either tin or plywood. I record everything I find underneath the covers, and I capture any reptiles I find underneath. Around 10 a.m. each day, I go to the lab to process the specimens that were caught the day before or earlier that morning.
Processing the animals involves collecting samples from each specimen. For turtles, Professor Goodman and I normally swab them and take a tissue sample from the tail by snipping a little bit off. For lizards and snakes we take tail tissue samples. We have a unique method for marking each squamate. We carve notches in the turtle shells, clip the lizard’s toes and we use a cauterizing tool to mark the snake’s scales on their underside. Since some of the reptiles might be infected with ranavirus, we are really careful during processing to keep everything disinfected.
Once the specimen has been processed, we return it to the area in which it was found. The majority of my work has been done in the field, and it has been a lot of fun. So far, I have caught 32 fence lizards, 9 five lined skins, 5 worm snakes, 20 Eastern Box Turtles, 1 ringneck snake and 1 rat snake. The time I spend out in the woods is very peaceful, and I have seen a lot of things that I will not soon forget.