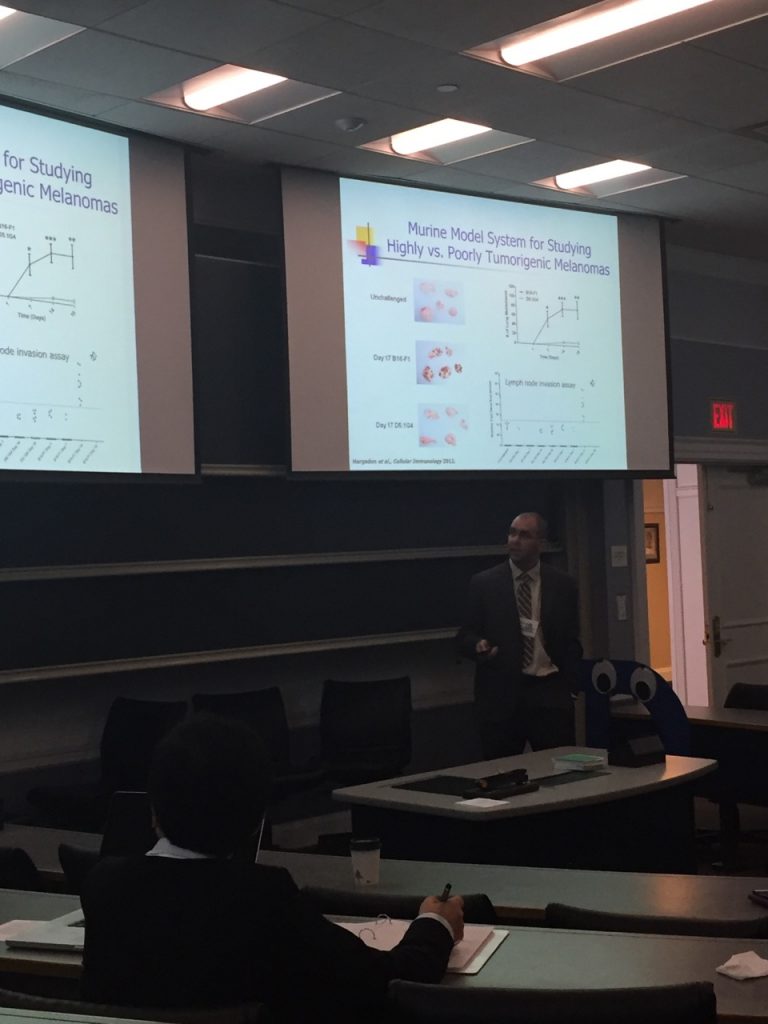
On Saturday Sept. 23 Elliott Associate Professor of Biology Dr. Kristian M. Hargadon ’01 joined more than 300 cancer researchers from major research universities across the state to present recent work from his laboratory at the inaugural Commonwealth of Virginia Cancer Research Conference held at the University of Virginia. Dr. Hargadon’s presentation focused on the role of the FOXC2 transcription factor as a critical driver of melanoma progression and featured work that he has conducted with 6 Hampden-Sydney College students over the last two years, all of whom were co-authors on the presentation. These student co-authors include Jefferson Thompson ’16 (applying to medical school), Travis Goodloe ’16 (now at University of South Alabama School of Medicine), James Lau ’17 (now at Eastern Virginia Medical School), Corey Williams ’19 (already accepted at Virginia Commonwealth University School of Medicine), Coleman Johnson ’19 (already accepted at Virginia Commonwealth University School of Medicine), and David Bushhouse ’19 (plans to pursue Ph.D. in a field of molecular genetics). Both Corey Williams ’19 and David Bushhouse ’19 joined Dr. Hargadon at the symposium and experienced firsthand the enthusiasm and momentum currently running through the field of cancer research. Major themes of the conference included new insights into molecular drivers of cancer progression and strategies to improve on the already promising targeted and immune-based therapies that are revolutionizing cancer treatment.
Author Archives: admin
Jamie Ingersoll ’18 Investigates Neuronal Synaptic Plasticity Abroad and at Home
My name is James Ingersoll ’18 and I’ve spent my summer working on a research study about the benefits of reading to your children. Before I talk about the research I’m working on this summer, I would like to talk about how I started my summer. The first day of my summer I was on a plane headed towards Madrid, Spain for a conference. I was fortunate enough to have the work I did during my 2016 H-SC summer research fellowship selected as a presentation poster at the Society for Neuroscience (SfN) in San Diego last November. I continued this work characterizing the neuronal changes that occur after fetal alcohol exposure by taking 6 credits of Biology Independent Research with Dr. Erin Clabough during the 2016-17 school year.
Jamie Ingersoll presents his work at the International Behavioural Neural Genetics Society Annual Meeting in Madrid
This last spring I was invited to the International Behavioural and Neural Genetics Society (IBANGS) conference in Madrid in May, so the day after graduation I was in Madrid attending the IBANGS conference as the only undergraduate there. I was a bit nervous going into the conference as I was the youngest attendee, but these nerves quickly subsided, as everyone at the conference was incredibly nice and personable. The conference was very different from SfN in San Diego, which was enormous. IBANGS was small, and everyone there were working on very interesting things, which was very cool to be able to hear more about. I had an incredible time in Madrid and am truly thankful for the young investigator travel award I received from IBANGS, as well as funding from H-SC, to allow conference attendance.
This summer, I’ve received a 2017 H-SC Summer Research Fellowship to continue this neuronal characterization, and to begin working on a research study that focuses on understanding better the benefits of reading to children, and if this reading alters the way children think or feel about others. Although these two projects may not seem related, both target synaptic plasticity, or the way that neurons can change in response to the environment. Sometimes these changes are negative, like in the case of early ethanol exposure, or they can be positive, like early exposure to reading.
Recruitment for this reading study has been a little tricky because a lot of parents are interested, but the children aren’t always as interested and if the kids say no, then I can’t enroll them into the study. It’s also been tricky because of vacation schedules. A lot of families have planned vacations that would interrupt the daily reading requirements of the study. Because of these factors, I haven’t been able to hit the numbers I intended on at the start of the study. These setbacks still don’t rival how much fun it’s been to work with children on something so interesting. I’m now working on running a statistical analysis on the data, and hope to have the stats done soon. I hope that my findings will prove to be useful for young families, help young kids be more school ready, and also allow us to learn more about synaptic plasticity.
Summer research on using biologically-inspired models to fight water pollution
By Brian Tarnai ’20
The opportunity to participate in summer research as a freshman was incredible. This research gave me my first real glance into how the scientific community really works. In my project I was working with both Hampden-Sydney and Virginia Tech professors to create a bioinspired, 3D printed prototype that would collect trash in rivers. The results of this work will be integrated into a National Science Foundation proposal that would enable undergraduates from across the nation to engage in projects that combine techniques in biology and engineering to ethically solve world challenges like water pollution.
During this project I stepped out of my comfort zone and developed valuable insight into many aspects of professional science that I was unaware of. I learned how to communicate with professors from different institutions to effectively accomplish a goal, write and submit research protocol, learn and master new technology, obtain permission to use live animals in an experiment, and how to correctly address adversity in a professional manner. This research has taught me that I have the ability to think big and also possess the practical means that will allow me to reach any goal I set. The lessons and skills that I’ve developed this summer will be extraordinarily beneficial on my path to medical school and into the world beyond.
H-SC students present class-based research at national HHMI symposium
2017 marks the sixth year of Hampden-Sydney’s participation in the Howard Hughes Medical Institute’s (HHMI) Science Education Alliance-Phage Hunters Advancing Genomic and Evolutionary Sciences (SEA-PHAGES) program. SEA-PHAGES is a national initiative in which undergraduates from roughly 100 institutions work to isolate and characterize bacteriophages, or viruses that infect bacteria, from the environment by molecular and bioinformatics methods. Each year, students from Dr. Mike Wolyniak’s Molecular and Cellular Biology course work to isolate and analyze novel bacteriophages while Genomics and Bioinformatics students annotate and study the DNA genomic sequences of these bacteriophages. Dakota Reinartz ’18 and Wood Morgan ’18 were members of both classes and recently presented some of the class’ work at the annual SEA-PHAGES symposium at HHMI’s Janelia Farm Research Campus in Ashburn, Virginia.
Dakota and Wood presented work on the class’ characterization of Thespis, a bacteriophage isolated previously by David Bushhouse ’19 that infects and destroys Mycobacterium smegmatis, a close relative of the bacteria responsible for tuberculosis.
The data collected from SEA-PHAGES students can be examined at phagesdb.org and has led to several peer reviewed publications on the ways in which viruses evolve and adapt in different environmental conditions. SEA-PHAGES is one of several opportunities Hampden-Sydney biology students get to interact with authentic research questions in their coursework.
Student summer research on whale behavior and baleen dynamics
By Mark Mason ’18
For my summer research project I am working with Drs. Alex Werth and Stan Cheyne from the Biology and Physics departments, respectively, to try and determine the factors that cause an unusual fish behavior. This behavior was first noticed when scientists observed humpback whales using bubbles to corral baitfish together near the surface, making them easier to catch, in a hunting strategy called bubble netting. Many studies have been done on the behavior of the whales and how they coordinate this unusual hunting strategy but there haven’t been any studies looking into why it works. This is why I will be using small fish and various tanks and aquarium bubblers to try and figure out why the fish don’t want to swim through the bubbles.
Eventually I will use the five hundred gallon tank in the physics department that Dr. Cheyne uses for his bubble research to try and replicate this phenomena. Early results in small freshwater tanks using zebrafish, a small species of minnow like fish, are promising and have proven that this is something that can be replicated in the lab.
By Shemar Blakeney ‘18
This summer, I am working with Professor Werth to examine the effects of oil on baleen plates. During these experiments, we will be analyzing the compression and tension of the baleen plates. Also, we will be using a flow tank to study the filtration system of baleen. With another version of the flow tank, we will test how the oil will affect the filtration system of the baleen plates. This research will benefit many baleen whales if an oil spill were to occur.
This research will benefit me in the future because I plan on obtaining my Ph.D. in Marine Biology and becoming a cetologist to study killer whales. The research I am doing is priceless to me.
Hampden-Sydney Summer Research Students Take On Melanoma
This summer, 3 rising H-SC juniors have been conducting melanoma research with Elliott Associate Professor of Biology Dr. Kristian M. Hargadon ’01. David Bushhouse, Coleman Johnson, and Corey Williams are investigating the role of the FOXC2 transcription factor as a driver of melanoma progression. Specifically, Corey has performed extensive phenotypic analyses of various wild-type and genetically engineered melanoma cells to study how FOXC2 expression in melanoma influences the expression of various integrins and other cell adhesion molecules on tumor cells. In related work, Cole has focused on studying how FOXC2 expression within these melanoma cells influences tumor cell adhesion to both the extracellular matrix and lymphatic endothelial cells, processes that are instrumental in determining whether or not a tumor is able to metastasize to distant sites in the body. In conjunction with this work, David is performing chromatin immunoprecipition studies to determine whether the regulation of cell adhesion molecule gene expression in melanoma cells that is driven by FOXC2 results from a direct interaction between this transcription factor and specific gene segments within the DNA of melanoma cells. Collectively, this work is shaping our understanding of FOXC2’s role in melanoma progression and has the potential to identify several potential targets for cancer therapies designed to interfere with the progression of this cancer. Cole and Corey were recently accepted into the Virginia Commonwealth University School of Medicine Early Selection Program, and David is planning to pursue a Ph.D. in the biomedical sciences.
Summer research in protein biochemistry
By Jason D. Pough II ’19
This summer I am working with Professor Michael Wolyniak to create an experiment for a laboratory that will be part of the future Biochemistry classes. Moreover, we are working on creating and purifying the Myf-5 Myogenic Regulatory Factor protein to assist with Professor Kristin Fischer’s research in regenerative skeletal muscle tissue as a part of creating the lab. Myf-5 is one of several Myogenic Regulator Factors that is involved with differentiating and creating muscle cells during Myogenesis. For Professor Fischer’s research, we plan to take a plasmid containing Mus musculus DNA and mutate it using site-directed mutagenesis in order to create a mutant Myf-5 protein that hopefully will aid in skeletal muscle regeneration research in conjunction with Professor Fischer’s research.
The overall project will aid in creating a Biochemistry laboratory by familiarizing ourselves with the techniques and methods used to carry out the Myf-5 experiment, and we will create methods and procedures for future biochemistry students to follow. One instance is with the aforementioned site-directed mutagenesis where, much like Real Time PCR, one uses primers and enzymes, yet we mutate specific sections of DNA. The mutated DNA is then inserted into a host bacterium where it will clone into a plentiful amount of bacteria with the mutated plasmid. Another instance is with the MinION Sequencer, provided by Oxford Nanopore, which uses thousands of protein pores to read the nucleotide sequence of injected DNA. The MinION Sequencer will be used to determine if the plasmids actually mutated and thus create a mutated protein. We hope that the results will not only aid Professor Fischer’s research, but also be the roots of future biochemistry laboratories and aid prospective Biology and Biochemistry and Molecular Biology majors.
H-SC summer research: developing scaffolds for mammalian tissue engineering
By Tyler McGaughey ’18
This summer I am working with Dr. Kristin Fischer to develop a porous gelatin cross-linked hydrogel scaffold for skeletal muscle tissue engineering. Through my work as an Emergency Medical Technician, I have seen numerous patients that have lost major sections of tissue. These injuries result from things like major trauma such as a car accident, violent crimes or systemic burns. These injuries have either forcefully removed the tissue or damaged it beyond repair. There are several clinical options doctors may choose: amputation, skin grafting, transplantation, or, the most interesting option, tissue engineering which is growing a new section of tissue in vitro. The ability to grow tissues outside of the body then implant them in or on humans used to be science fiction, but it is happening this summer on “The Hill”.
Dr. Fischer and I are working to answer the question of what is the best way to use the tissue engineering approach. Currently we are working with a line of mouse muscle cells called C2C12.
The cells grow well given the right environment in flat sheets. However, the problem stems from layering the cells vertically. The cells in the center of the mass begin to die off due to lack of nutrients and surface area for diffusion of waste products. I intend to solve this problem by developing a gelatin scaffold for the cells to grow in. This will allow for increased diffusion and hopefully increase cell longevity.
I plan to increase diffusion to the gel by introducing pores in varying configurations. Over the last two weeks, I have tried varying number of pins per scaffold from 0-12 pins per scaffold. I have also experimented with different shapes like diagonal lines, squares, and triangles. I have concluded that the triangle formation is most likely the best formation for diffusion. I am currently attempting to print these pore inducing structures using HSC’s newest 3D printer.
The cross-linking helps the gelatin maintain its 3D structure. Cross-linking is the binding of gelatin molecules together by an enzyme called microbial transglutaminase. I have also been experimenting with different levels of microbial transglutaminase in the gelatin. More cross-linking makes the gels stiffer. There is a fine line between too much microbial transglutaminase causing the gels to rip under tension and too little microbial transglutaminase causing the gelatin to degrade too quickly.
In the body, muscle cells fuse together and work as one. This fusion is caused by the natural tension our muscle cells are under. In addition to introducing pores into the gel, I intend to apply a slight tension to the gels. This tension causes the muscle cells to fuse and mature in one direction as if they were in the body.
Hopefully this summer I am able to design a gelatin scaffold that helps muscle cells grow rapidly and mature.
3D Printing comes to H-SC Biology
As part of a seed grant project between Hampden-Sydney and Virginia Tech for the development of research opportunities in bioengineering, the Biology Department has received a 3D printer for classroom and research purposes. As the summer research season kicks off at the College, the timing of this new acquisition could not be better:
The first student project that will take place using the printer will be the construction of filter structures that can be used to clean local streams and lakes. The project is well-positioned for both undergraduate research and extension to the Department’s ongoing high school outreach program.
Congratulations to the Biology Class of 2017!
The Biology Class of 2017 has achieved great things in their four years at the College, and we will miss their presence in Gilmer Hall. Best of luck to all of our graduates!
DJ Bines Fletcher Borum
Brant Boucher Blake Brown
Robbie Bugbee Josh Chamberlin
Alex Crabtree Tazewell DelDonna
William Echols Gannon Griffin
Treavor Hartwell Ryan Kluk
James Lau Zach Martin
Traylor Nichols Tyler Reekes
Reuben Retnam Zach Tabrani
Harris Thomas Mitchell Thomas
Joey Tyler Thomas Vinyard
Dustin Wiles Michael Willis
A.J. Willy John Zohab